











18 Spectroscopic Analyzer Manufacturers in 2024
This section provides an overview for spectroscopic analyzer as well as their applications and principles. Also, please take a look at the list of 18 spectroscopic analyzer manufacturers and their company rankings. Here are the top-ranked spectroscopic analyzer companies as of April, 2024: 1.Labsphere, Inc., 2.Mightex Systems, 3.AMETEK Process Instruments.
Table of Contents
What Is a Spectroscopic Analyzer?
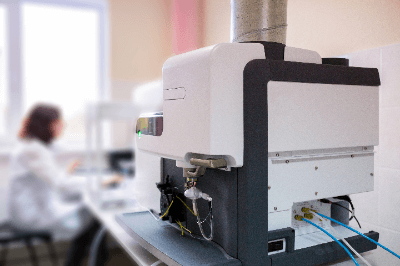
A spectroscopic analyzer is a versatile analytical instrument used to examine the spectrum of light emitted or absorbed by substances. These devices typically consist of a light source, a spectroscopic section, a sample section, and a detector.
Spectroscopic analyzers are categorized based on their light source and operational mechanism. Notable types include ultraviolet-visible spectrophotometers (UV-Vis), infrared spectrophotometers (IR), inductively coupled plasma atomic emission spectrometers (ICP-AES), atomic absorption spectrometers (AAS), fluorescent X-ray spectrometers (XRF), and X-ray photoelectron spectrometers (XPS). Each instrument serves specific analytical purposes due to its distinct capabilities.
These analyzers are indispensable tools for both qualitative and quantitative analysis of samples in various fields, including research, quality control, and chemical analysis.
Types of Spectroscopic Analyzers
Spectroscopic analyzers can detect the light emitted or absorbed by substances, enabling a wide range of analyses. Here are six representative types of spectroscopic analyzers:
1. Ultraviolet-Visible Spectrophotometer (UV-Vis)
This instrument utilizes ultraviolet or visible light sources to examine light transmitted through or reflected from a material. It facilitates qualitative and quantitative analysis of sample components.
2. Infrared Spectrophotometer (IR)
IR spectrophotometers employ infrared light sources to study light transmitted through or reflected from materials. They are used to estimate molecular structures and perform quantitative analysis of sample components.
3. Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES)
This device detects luminescence generated when a sample is introduced into an inductively coupled plasma. It offers exceptional sensitivity and is ideal for qualitative and quantitative analysis of trace elements.
4. Atomic Absorption Spectrometer (AAS)
AAS instruments perform qualitative and quantitative analysis of trace elements by measuring the absorption of specific wavelengths of light by atoms.
5. X-Ray Fluorescence Analyzer (XRF)
XRF analyzers conduct elemental analysis of materials using X-rays as the light source. They identify qualitative and quantitative characteristics by observing the fluorescent X-rays specific to each element.
6. X-Ray Photoelectron Spectrometer (XPS)
XPS devices employ soft X-rays as the light source to gather information about the atoms and molecules on the surface of a solid material.
Spectroscopic Analysis
Spectroscopic analysis involves the examination of a substance's properties by shining light on it and observing its response to the light. The fundamental principle behind this analysis is that materials interact with light in specific ways, allowing us to gather information about them.
For instance, when light illuminates a substance, it can be absorbed, reflected, or emitted at specific wavelengths. Spectrometers are designed to capture and analyze these interactions. By studying the resulting data, various types of information can be extracted, including qualitative and quantitative analysis of samples, molecular structure evaluation, and material property assessment.
The key examples include:
- The evaluation of the electronic states of molecules using ultraviolet and visible wavelengths.
- The determination of atomic and molecular arrangements on the surface of solids using X-ray spectra.
- The analysis of vibrational states between atoms in organic compounds through infrared spectra.
Spectrum in Spectroscopic Analysis
In spectroscopic analysis, the spectrum is a crucial element that provides material information. It is obtained by recording the light intensity at various wavelengths using a spectrometer. The resulting spectrum consists of peaks and troughs, each with a specific meaning depending on the type of spectrometer used.
1. UV-Visible Spectrophotometer
The spectrum often appears as a mountain-shaped curve, with peaks indicating the wavelengths at which electrons enter excited states.
2. Infrared Spectrophotometer
The infrared spectrum can exhibit both broad peaks and sharp lines. The presence of specific peaks reveals the vibrations between atoms in organic compounds.
3. Inductively Coupled Plasma Emission Spectrometer
This instrument detects luminescence emitted when a sample is exposed to inductively coupled plasma. The emitted energy, converted to wavelengths, appears as peaks in the spectrum.
4. Atomic Absorption Spectrometer
Atomic absorption spectrometers identify specific wavelengths of light absorbed by atoms in primarily inorganic substances. These absorbed wavelengths are evident as peaks in the spectrum.
5. X-Ray Fluorescence Analyzer
Similar to luminescence, XRF analyzers record the wavelengths of fluorescent X-rays emitted when electrons return to their ground state following X-ray irradiation, representing peaks in the spectrum.
6. X-Ray Photoelectron Spectrometer
XPS instruments utilize soft X-rays to ionize atoms or molecules on a solid surface, and the emitted electrons' energy is detected as peaks in the spectrum.
List of 18 Spectroscopic Analyzer Manufacturers
*Including some distributors, etc.
Sort by Features
- Default
- Company Size: largest first
- Year Founded: oldest first
- Year Founded: earliest first
Sort by Area
- United States of America
- Canada
- Japan
- United Kingdom
-
-

-
International Light Technologies, Inc.
Spectrometers
Manufacturer Overview
International Light Technologies was established in 1965 as a manufacturer of light-measuring instruments based in Peabody, Massachusetts. The company provides light measurement products that include light meters, spectrophotometers, radiometers, and spectroradiometers for applications that require measuring of UV, visible light, and of infrared. There are also other accessories such as detectors, filters, calibrations, and integrating spheres that are used along with the main products. The products can withstand harsh environments such as underwater applications and have extensive use in medical, scientific, and industrial manufacturing processes.
-
-
-
-

-
Broadcom
Spectrometers
Manufacturer Overview
Broadcom Inc., established in 1961, and headquartered in San Jose, California, is a manufacturer and supplier of semiconductor and connectivity solutions. The company specializes in producing products, such as semiconductors, infrastructure software, networking products, storage products, wireless products in which their products cater to industries such as telecommunications, data centers, and consumer electronics. Their products are used for various applications, including wireless communication, networking equipment, data storage, and multimedia devices, providing components for advanced technologies in numerous industrial and commercial sectors.
-
-
-
-

-
Labsphere, Inc.
CDS Spectrometers
Manufacturer Overview
Labsphere, established in 1979, is headquartered in North Sutton, New Hampshire, United States, and the company specializes in developing and producing advanced light measuring technologies tailored for the LED/SSL lighting sector. The company produces light measuring equipment designed for many industries like aerospace, automotive, electronic imaging, laser diode, LED, lighting, medical imaging, and optics. The company provides clients with a range of advanced technological solutions, including pulsed laser power measurement systems, ultraviolet light-emitting diode characterization systems, high-temperature spectroradiometers, tunable portable luminance test and calibration reference sources, and ultraviolet transmittance analyzers.
-
-
-
-

-
Spectral Products
Spectrometers
Manufacturer Overview
Spectral Products LLC is an American manufacturer based in Putnam, Connecticut, specializing in optical instrumentation technology, Formerly a subsidiary of CVI Laser Optics LLC, an American optical component manufacturer, Spectral Products introduced the first microprocessor-controlled monochromator in 1987. They expanded their product line with Digikröm, a combination of precision optical mechanics and microcomputer technology. The company develops computer-based spectrometers for spectral measurement in the ultraviolet (UV), visible, and near-infrared (NIR) ranges. Furthermore, they offer industrial light sources and filter wheels as part of their optical instruments. The company’s products find applications across various industries, including the environmental, biomedical, mining, and pharmaceutical sectors.
-
-
-
-

-
Ocean Insight
Spectrometers
Manufacturer Overview
Ocean Insight is the Applied Spectral Knowledge company, founded in 1989, is a manufacutring company using spectral technology, application expertise, and manufacturing scalability to help customers take on important challenges for a safer, cleaner, healthier future, headquartered in Florida, USA. The company consists of three industry brands: Ocean Optics, which pioneered miniature spectrometers and delivers spectral solutions to researchers, OEMs and industrial customers; Ocean Applied, which designs and builds industrial-grade photonics systems for material inspection, chemical identification and quality assurance; and International Light, which supplies lighting assemblies, light measurement solutions and calibration services.
-
-
-
-
-
Kristen Mann Design LLC
Spectrographs
Manufacturer Overview
Spectra Solutions Inc. (SSI) is a manufacturer of spectroscopic analytical and Raman instruments based in Massachusetts. The company not only deals in spectrographs, but the fiber optic Raman probes are utilized by industrial companies and scientific research institutions worldwide as the focusing lens barrel in it is interchangeable, and the Raman Monitoring System (RAMS) has the capability to monitor up to 6 probes simultaneously. Lasers and microscopes are also listed as products supplied by the company, helping to serve the pharmaceutical, chemical, biochemical, petroleum, nuclear, and similar industries.
-
-
-
-

-
Advion, Inc.
spectroscopy
Company Overview
Advion is a manufacturer and supplier of mass spectrometry and ICP-MS solutions based in New York. The company provides chemist-centric, purpose-built mass spectrometers, nanoelectrospray ionization sources, and ICP-MS solutions for elemental analysis used in chemical biology and other applications and has collaborated with Interchim SAS, to establish Advion Interchim Scientific, an international frontrunner in pioneering instruments and solutions designed for applications in synthesis, life sciences, and analytical and purification processes utilizing chromatography. These solutions have various applications in clinical, pharmaceutical, environmental, and mass spectrometry analysis of compounds and elements and the food and ingredient sectors.
-
-
-
-

-
StellarNet, Inc.
SpectroRadiometer System Features
Company Overview
StellarNet, Inc. is an American miniature spectrometer system products manufacturer and supplier that was established in Tampa, Florida, in 1991. The company’s product lineup includes portable and compact fiber optic spectrometers for near-infrared (NIR), visible (VIS), and UV measurements. It also offers various specialized light sources such as deuterium, tungsten halogen, and mercury argon. The company’s products are commonly used in academic and research institutions, as well as in the medical industry.
-
-
-
-

-
Bentham Instruments Limited
Single Monochromator
Manufacturer Overview
Bentham Instruments Limited is a British manufacturer of light measurement instruments founded in 1975 and based in Reading, Berkshire. The company produces standard as well as custom instruments and equipment for studying or measuring lights. These include variable radiance uniform light sources, fiber spectral attenuation spectrometers, and source characterization systems. Aside from its products, the company also offers OEM as well as calibration services for customers in the biomedical, nanotechnology, and research sectors needing additional support.
-
-
-
-

-
APEL Co.,Ltd.
Analytical Equipment
Manufacturer Overview
APEL Co., Ltd., a company established in 1976 and headquartered in Saitama, Japan, is a developer and manufacturer of medical devices for clinical laboratories. The company specializes in the production of bilirubin meters, hemoglobin meters, coagulometers, and hematocrit centrifuges, used in all fields of analysis of liquid concentration, such as the Department of Obstetrics and Gynecology, blood centers, laboratories, and food and beverage manufacturers. The company also provides customer services, including technical support and customization options, to meet customer needs.
-
-
-
-
-
-

-
LIGHTMACHINERY, INC.
WEAK SOURCE SPECTROMETERS
Manufacturer Overview
LightMachinery, Inc., established in 2002 and headquartered in Ottawa, Canada, is a manufacturer specializing in the development of precision laser solutions for a wide range of industries. In 2009, the company made a significant stride by acquiring GSI Lumonics' pulsed gas laser product lines. These lines were renowned for their established capability for fast, on-the-fly marking of pharmaceutical, electronic, and consumer packaging products. The company's portfolio of main products includes advanced laser systems, optical components, and laser micro-machining tools. The laser systems are designed to deliver precision in several applications, such as material processing, engraving, and medical device manufacturing.
-
-
-
-

-
IAS Analytics
spectrometers
Manufacturer Overview
IAS Analytics, headquartered in Los Angeles, California, is a manufacturer and supplier of near-infrared (NIR) spectroscopy instruments and solutions. The company's products includes NIR spectrometer, portable grain analyzer, and NIR spectrum analyzer, which caters to various industries such as oil, food, dairy, wine & beer, and meat industries. Its products are employed for rapid and non-destructive material analysis, such as determining composition, quality, and authenticity of agricultural products, pharmaceuticals, and chemicals.
-
-
-
-

-
AMETEK Process Instruments
spectrometers
Manufacturer Overview
AMETEK Process Instruments, was founded in and is currently headquartered in Pittsburgh, Pennsylvania as a business unit of the Process & Analytical Instruments division of AMETEK, Inc. The company is a manufacturer and engineer that serves customers in markets such as power generation, pollution monitoring, glass manufacturing, semiconductors, and fuel cell research. The company designs analyzing equipment for customer-specific applications. The company manufactures under brands such as Chandler Engineering which produces gas gravitometers, Process Instruments, THERMOX, Trace Analytical, and Western Research.
-
-
-
-

-
World Star Tech Inc.
spectrometers
Company Overview
World Star Tech Inc., established in Markham, Ontario, in 1996 is a manufacturer and distributor of laser diode modules, solutions and instrumentation. The company's product portfolio includes Benchtop Lasers, Line Laser Modules, Cross Laser Modules and TEC Laser Systems Fiber Coupled Laser Systems. Their products are used for research, manufacturing, laser measurement and control instruments for accurate performance. The company serves industries including Biotechnology and Medical, Aerospace and Defense. The company also offers industry based custom solutions, customer support and technical service.
-
-
-
-

-
PCE Instruments UK Ltd
spectrometers
Company Overview
PCE Instruments UK Ltd., started in 199 and headquartered in Manchester, UK, is a manufacturer and supplier of test instruments, equipment, and tools for weighing, measuring, and control systems. The company offers more than 500 test equipment, including analyzers, inspection cameras, meters, detectors, and sensors, with applications in various fields like data acquisition, electrical engineering, environmental science, building inspection, and food processing. Its manufacturing and development division is ISO 9001 certified, all its test instruments, equipment, and tools are factory calibrated, and the company also provides services for custom test instrument design, installation, and maintenance.
-
-
-
-

-
Mightex Systems
Spectrometers
Company Overview
Mightex is a manufacturer and supplier of optical stimulation & imaging tools based in Toronto, Canada since 2005. The company deals in all-optical cellular-resolution optogenetics, optical simulation systems, and Bioscience imaging tools and software for data acquisition and analysis helping with life science research. Some of the equipment includes a Polygon DMD pattern illuminator, microscopy LEDs, Fluorescence microscopy cameras, and more supporting applications of neuroscience, optogenetics, live cell imaging, and more in the field of biosciences.
-
-
-
-

-
Due2Lab s.r.l.
HXS
-
-
Spectroscopic Analyzer Manufacturer Ranking
*Including some distributors, etc.Ranking as of April 2024
Derivation Method| Rank | Company | Click Share |
|---|---|---|
| 1 | Labsphere, Inc. |
16.6%
|
| 2 | Mightex Systems |
10.0%
|
| 3 | AMETEK Process Instruments |
7.7%
|
| 4 | Spectral Products |
7.3%
|
| 5 | International Light Technologies, Inc. |
6.9%
|
| 6 | Ocean Insight |
6.6%
|
| 7 | StellarNet, Inc. |
6.6%
|
| 8 | World Star Tech Inc. |
5.8%
|
| 9 | LIGHTMACHINERY, INC. |
5.4%
|
| 10 | Advion, Inc. |
4.6%
|
Derivation Method
The ranking is calculated based on the click share within the spectroscopic analyzer page as of April 2024. Click share is defined as the total number of clicks for all companies during the period divided by the number of clicks for each company.Number of Employees
- Shimadzu Seisakusho Co., Ltd.: 13,499
- Ocean Insight: 200
Newly Established Company
- World Star Tech Inc.: 1996 (28 years ago)
- StellarNet, Inc.: 1991 (33 years ago)
- Labsphere, Inc.: 1979 (45 years ago)
Company with a History
- Shimadzu Seisakusho Co., Ltd.: 1875 (149 years ago)
- Bentham Instruments Limited: 1975 (49 years ago)
- APEL Co.,Ltd.: 1976 (48 years ago)
Spectroscopic Analyzer Manufacturers in United States
*Including some distributors, etc.
- International Light Technologies, Inc.
- Broadcom
- Labsphere, Inc.
- Spectral Products
- Ocean Insight
- Kristen Mann Design LLC
- Advion, Inc.
- StellarNet, Inc.
Global Distribution of Spectroscopic Analyzer Manufacturers by Country
*Including some distributors, etc.
| Country | Number of Companies | Share (%) |
|---|---|---|
 United States of America
United States of America
|
8 | 57.1% |
 United Kingdom
United Kingdom
|
2 | 14.3% |
 Japan
Japan
|
2 | 14.3% |
 Canada
Canada
|
2 | 14.3% |
List of Spectroscopic Analyzer Products
2 products are listed.
Heraeus Holding GmbH
Realize a broad wavelength in the ultraviolet area Fiberlight® L3
LED light sources are also developed in the ultraviolet region, and it is a light source with excellent power saving, stability, and response speed...
Quality Design Co., Ltd.
High -speed and non -destruction / non -contact measurement real -time near infrared division light unit Luminar series
Near infrared division unit for pharmaceutical companies ■ Overview The "LUMINAR Series", which is handled by the quality design, quickly feeds th...

